RENOVATION, STANDARD LOOK
RENOVATION, STANDARD LOOK

WAKAMONO SURGICAL MASK
FDA, CE & TGA CERTIFICATE
WAKAMONO SURGICAL MASK met and exceed, the ASTM F2100 – Level 2/3 and EN 14683 – Type IIR
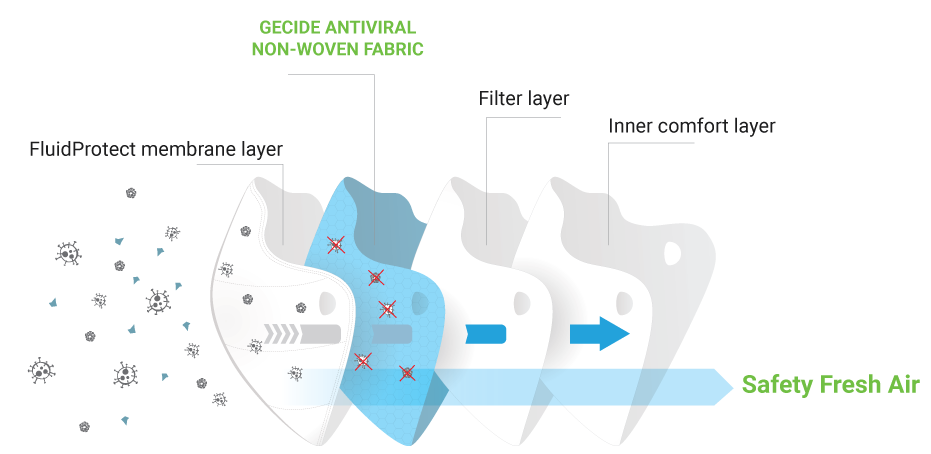
The World’s first surgical mask applying Nano Biotech to proven effective against 99% SARS-CoV-2 and HUMAN CORONAVIRUS. WAKAMONO Surgical Mask applied GECIDE fabric not only deactivates INFLUENZA A H1N1 – the enveloped viruses but also inactivate POLIOVIRUS-I – the non-enveloped viruses.
The test performance was tested directly on viruses and certified by reputable independent laboratories according to the standard ISO 18184:2019.

(1) Anti-HUMAN CORONAVIRUS test – standard ISO 18184:2019 – TÜV SÜD.
(2) Anti-INFLUENZA A H1N1 virus and Anti-POLIOVIRUS-I virus test – standard ISO 18184:2019 – Guandong Detection Center of Microbiology.
(3) Anti-SARS-CoV-2 test – standard ISO 18184:2019 – Independent Laboratory in India.
(4) Anti-HUMAN CORONAVIRUS test – standard ASTM E1052 – EUROFINS
(5) Time kill test – Long-lasting bactericidal activity – Bureau Veritas.
(6) Biological evaluation – ANSI/AAMI/ISO10993-5:2009 – Pacific Biolabs Inc.
(7) ASTM F2100 Level 2/3, EN 14683 Type IIR – TÜV SÜD.
(8) Antibacterial efficiency test – AATCC 100:2019 – Quality Assurance and Testing Center 3.